Check out our full list of publications
Multiplexed Live-Cell Imaging for Drug Responses in Patient-Derived Organoid Models of Cancer

Patient-derived tumor organoids are a sophisticated model system for basic and translational research. This methods article details the use of multiplexed fluorescent live-cell imaging for simultaneous kinetic assessment of different organoid phenotypes.
Enhancing Progestin Therapy with a Glucagon-Like Peptide 1 Agonist for the Conservative Management of Endometrial Cancer
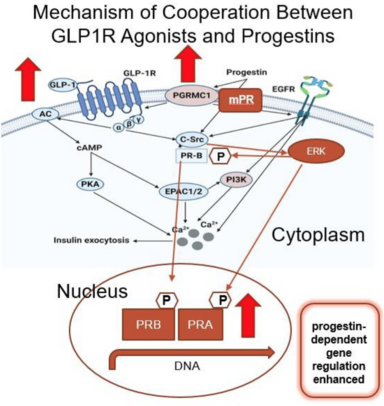
Since obesity is a major risk factor for endometrial cancer, we explored a dual treatment strategy by combining a weight loss drug (semaglutide—a glucagon-like peptide-1 (GLP-1) agonist) with a progestin. Progestins are a safe and highly effective non-surgical treatment strategy for early-stage/grade endometrial cancer, but the response is often not sustained. Using cell lines and patient-derived preclinical models of endometrial cancer, we discovered an unexpected interplay between the GLP-1 receptor and progesterone receptor. Specifically, GLP-1 agonists increased expression of the progesterone receptor, and the combination of the two agents resulted in more pronounced cell death as compared to a single agent alone. As more patients are exploring GLP-1 agonists for weight management, these data make an important contribution to the field by suggesting that such drugs can also increase the efficacy of hormone therapy in endometrial cancer.
Global expression analysis of endometrial cancer cells in response to progesterone identifies new therapeutic targets
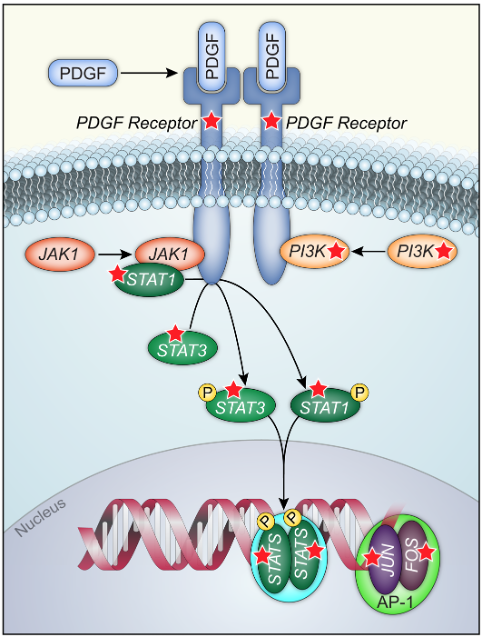
Progesterone prevents development of endometrial cancers through its receptor (PR) although the molecular mechanisms have yet to be fully characterized. In this study, we performed a global analysis of gene regulation by progesterone using human endometrial cancer cells that expressed PR endogenously or exogenously. We found progesterone strongly inhibits multiple components of the platelet derived growth factor receptor (PDGFR), Janus kinase (JAK), signal transducer and activator of transcription (STAT) pathway through PR. The PDGFR/JAK/STAT pathway signals to control numerous downstream targets including AP-1 transcription factors Fos and Jun. Treatment with inhibitors of the PDGFR/JAK/STAT pathway significantly blocked proliferation in multiple novel patient-derived organoid models of endometrial cancer, and activation of this pathway was found to be a poor prognostic signal for the survival of patients with endometrial cancer from The Cancer Genome Atlas. Our study identifies this pathway as central to the growth-limiting effects of progesterone in endometrial cancer and suggests that inhibitors of PDGFR/JAK/STAT should be considered for future therapeutic interventions.

